Documents
· Screening form· Official info
· Info for participants
· Publication (Quantitive)
· Publication (Longitudinal)
Investigators:
Dr. A. Wilman
Dr. G. Blevins
INFORMATION ON HIGH FIELD MRI STUDY ON MULTIPLE SCLEROSIS
Main study info | Info for participants | Contact info | Preliminary results | Media
For this research study, we use a stronger MRI
than that used in the clinic. This allows us to obtain images with higher
resolution and with more sensitivity. We do not use any contrast agents for this study.
Project: Evaluation of MRI in normal volunteers and patients with suspected neurological disease
Investigators: Alan H. Wilman, PhD, Derek J. Emery MD FRCPC, Gregg Blevins MD FRCPC.
Background: Magnetic resonance imaging or "MRI" is a method of painlessly looking inside the body without x-rays. MRI uses a very strong magnet and radio waves to produce detailed images.
Purpose: This research is being done to see how well we can image the brain and blood vessels supplying the brain.
Procedures: We are asking you to undergo an MRI examination. The MRI exam itself is painless. The examination involves lying on your back in the MRI machine for about an hour. While in the MRI machine you will be in a long hollow cylinder that is 60 cm wide and about 3 meters long. Your head will go into a smaller cylinder that will be about 6 cm (2 inches) from your face. You will hear a banging or knocking sound as the computer opens and closes various switches. We will supply ear plugs to reduce the noise. You will also be given a small hand-held alarm so that you can stop the procedure at any time.
Possible benefits: This study may help us develop better ways of seeing the brain.
Possible risks: The MRI exam has no known harmful effects.
Confidentiality: Only the people conducting this study will see the information obtained. We will not give your name to anyone outside the study.
Withdrawal: You may withdraw from the study at any time. Your care, and that of your family, will not be affected if you do not participate in this study.
If you have concerns about any part of this study, you may contact the Patient Concerns Office of the Capital Health Authority at 407-1040. This office has no affiliation with the study investigators.
Information for participants
Because of the higher field, you cannot have had previous surgery where you may have had metal put in your body (from staples, clips, etc). Please fill out the pink (female) or blue (male) screening form.If you check "yes" to any of the questions, it is possible that you cannot undergo a high-field MRI scan. If you check "yes", please call the research MRI coordinator to assess your eligibility. Otherwise, if you have not had surgeries and have checked "no" to all questions, just bring this form at the time of your appointment.
For the procedure, you will be asked to change into provided hospital pants and shirt, remove all jewelry and anything else that may contain metal (eg underwire bra). The procedure takes less than an hour, with a little extra time needed to fill out the paperwork and change.
How to find us:
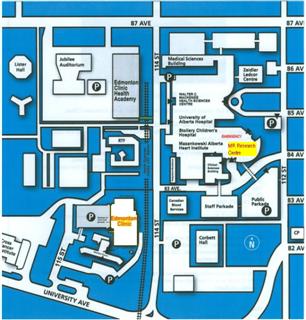
Please follow the directions to the MR Research Centre waiting room (0A6.11), located in the basement of Mazankowski Heart Institute (8440 112 Street), the building east of the MS clinic.
- Enter MHI from 84 Avenue (either main floor, or 2nd floor which has direct walkway to parkade)
- Take elevators down to basement level (elevators are hidden near south-east facing wall)
- Turn RIGHT out of elevators, proceed through double (brown) doors and follow signs to "MR Research Centre" (about 50m walk)
- Enter RED doors of the MR Research Centre and proceed to the patient waiting area (on the right hand side)
Parking reimbursement provided:
$40 cash
Contact information:
Research MRI coordinator (screening, appointments):Information about the study in general:
MRI Research Lead
Dr. Alan Wilman - 780-492-0562 (also a private line).
Preliminary results
Quantitative high field imaging of sub-cortical gray matter in multiple sclerosis.
Lebel RM, Eissa A, Seres P, Blevins G, Wilman AH.
Mult Scler. 2011 Oct 27.
Abstract (Please contact Dr. Wilman or Mr. Seres to obtain the complete publication)
Background: In addition to neuronal injury, inflammatory, and demyelinating processes, evidence suggests multiple sclerosis (MS) is also associated with increased iron deposition in the basal ganglia. Magnetic resonance imaging (MRI), particularly at very high field strengths, is sensitive to iron accumulation and may enable visualization and quantification of iron associated with MS.
Results: Significant abnormalities were observed in most structures, most notably in the pulvinar sub-nucleus. Significant correlations with disability were observed in the pulvinar; marginally significant correlations were also observed in the thalamus and red nucleus. No significant correlations were observed with duration since index relapse.
Conclusions: Widespread abnormalities are present in the deep gray matter nuclei of patients recently diagnosed with MS; these abnormalities can be detected via multi-modal high-field MRI. Imaging metrics, particularly R2*, relate to disease severity in the pulvinar and other gray matter regions.
Longitudinal MR Imaging of Iron in Multiple Sclerosis: An Imaging Marker of Disease.
Walsh AJ, Blevins G, Lebel RM, Seres P, Emery DJ, Wilman AH.
Radiology. 2013 Aug 7.
Abstract (Please contact Dr. Wilman or Mr. Seres to obtain the complete publication)
Purpose:To investigate the relationship between magnetic resonance (MR) imaging markers of iron content and disease severity in patients with multiple sclerosis (MS) over a 2-year period.
Results:R2* mapping using 2-year-difference measurements had the highest correlation to disease severity (r = 0.905, P < .001) compared with R2* mapping using single-time measurements (r = 0.560, P = .019) and phase imaging by using either single-time (r = 0.539, P = .026) or 2-year-difference (r = 0.644, P = .005) measurements. Significant increases in R2* occur during 2 years in the substantia nigra (P < .001) and globus pallidus (P = .035), which are both predictors of disease in regression analysis, in patients compared with control subjects. There were group differences in the substantia nigra, globus pallidus, pulvinar thalamus, thalamus, and caudate nucleus, compared with control subjects with R2* mapping (P < .05), and group differences in the caudate nucleus and pulvinar thalamus, compared with control subjects with phase imaging (P < .05).
Conclusion:There are significant changes in deep gray matter iron content in MS during 2 years measured with MR imaging, changes that are strongly related to physical disability. Longitudinal measurements may produce a higher correlation to disease severity compared with single-time measurements because baseline iron content of deep gray matter is variable among subjects.
Media
University of Alberta News, December 15, 2011
MRI power to track MS
Medical researchers at the University of Alberta have discovered a new way to track the progression of multiple sclerosis by using a powerful, triple-strength MRI to assess increasing levels of iron in brain tissue.
http://www.news.ualberta.ca/article.aspx?id=9A30BD41D9F84624865677B7B70E849D
http://www.youtube.com/watch?v=ebdOXZ2cAG8
GlobalTV
MS Society Newsletter, March 2012 (page 10)
(local copy)